Perhaps one of the oldest products of man’s chemical manipulation, soap has been known for over 3000 years. According to legend, soap was discovered at the base of a hill called Mount Sapo. There, animals were sacrificed on altars and burned over wood fires. The grease from the animals mixed with the wood ashes ran down the hill to the riverside, where women discovered its cleansing power while washing clothing in the river.
Saponification, the process that leads to the formation of soap from fats and oils, is named for the hill on which it was discovered. Interestingly, the process of making soap remained relatively unchanged for centuries. You may have heard of pioneers in the old west who made their own “cakes” of soap from animal fat and potash or lye.
What is the chemistry of soap?
Soaps are the salts of fatty acids and have chemical structures analogous to the figure at the right. Because soap is the salt of any fatty acid, and there are many fatty acids of varying structures, the properties of soap differ depending on the fats or oils used to make it.
Soap can be produced by reacting lard (animal fat) with NaOH or KOH. Recall that these “fats” (particularly those of animal origin) are predominantly triglycerides containing three fatty acids esterified to one glycerol molecule. When triglycerides are boiled in an aqueous solution of a strong base, the molecules are cleaved (broken) into glycerol and fatty acid components.
Solutions of either NaOH or KOH can be used for this reaction; however, the properties of the resulting soaps are different. Sodium soaps have higher melting points and are generally solid at room temperature. Hence, sodium salts are used for bar soaps. Potassium soaps are usually liquids at room temperature and are more commonly used for liquid soaps.
It is essential to realize that many sources of fats and oils are characterized by a unique distribution of fatty acids. For example, olive oil contains primarily oleic acid, an eighteen-carbon unsaturated fatty acid, whereas palm oil contains both oleic acid and palmitic acid, a sixteen-carbon saturated fatty acid.After the strong base has reacted with the oil, the soap formed is separated from the reaction mixture by increasing the concentration of Na+ or K+ in the solution. Because soaps exhibit reduced solubility in the presence of high Na+ and K+ concentrations, the soaps precipitate and form a “cake.” This process is known as salting out.
At this point, the soap can be filtered from the glycerol and the excess base that remains in the reaction mixture. Facial and bath soaps are highly purified to remove any excess base that may be abrasive to sensitive skin. For this reason, soap produced in the laboratory should never be used to cleanse the body.
Are commercial soaps better than self-made?
Commercial soaps often contain additives such as fragrances, dyes, and antimicrobial agents; however, these do not improve the cleansing power of the soap, only the appearance and consumer appeal. Some bars of soap float. This is achieved by incorporating air bubbles into the bar as it is molded.
The cleansing power of soap is determined by its solubility. To clean effectively, soap must form suds readily in water. Soaps made from fatty acids with short carbon chains are more water soluble than those with long ones.
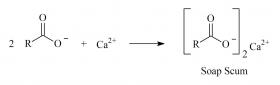
You may have learned by experience that soap does not clean well in hard water. Water is considered hard when it contains high concentrations of magnesium, calcium, and iron ions. These cations replace the sodium or potassium ions in soap and form insoluble precipitates commonly referred to as soap scum.
The formation of soap scum removes available soap molecules from the solution, decreasing its ability to clean. In addition, soap scum can form films of residue in bathtubs and shower stalls. Below is the reaction for the formation of soap scum, where ‘R’ is shorthand for the long carbon chain of the soap molecule.Many commercial soaps contain additional chelating agents that form complexes with metal ions in hard water and increase the cleansing efficiency of a product. However, chelating agents are accessible, so experienced soap-makers can add them to the formula and produce effective self-made soaps useful with hard water.
What are micelles?
Soaps are used for cleaning because their long hydrophobic carbon tails dissolve well in nonpolar materials such as grease, chocolate, grime, and oils. Soaps dispersed in water naturally form micelles, spherical aggregates of hundreds of soap molecules oriented with the long carbon chains in the interior and the polar head groups on the exterior. For this reason, micelles remain soluble in water, even when they contain additional oils (e.g., from “dirt”) in their interiors.