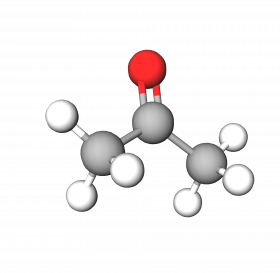
Acetone occurs naturally in trees, plants, decomposing vegetation, and volcanic gas. Our body produces small amounts during the breakdown of fat as well. However, the majority of acetone is manufactured for industrial uses from benzene and propylene. Also known as dimethyl ketone, it is a flammable, clear liquid that is an effective solvent as well as an additive in commercial manufacturing.
The diverse uses of acetone include unlikely places such as particle boards, man-made fibers, shoes, waxes, printer ink, and film. Most importantly, it serves as an intermediate for producing other chemicals.
Commercially, acetone is present in BPA production (Bisphenol A) which is then used to create epoxy resins, and it is a key ingredient in making acrylic plastics found on signs and light fixtures. It is best known for its strength as a solvent, however. Acetone accounts for the strong odor of nail polish remover, superglue, varnish, lacquer, and paint remover to name a few.