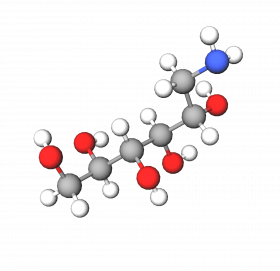
Glucamine is an organic primary amine, which has a sugar linked to its structure. It is used for the neutralization of carbomers and acrylate polymers, making it possible to obtain transparent and stable cosmetic and pharmaceutical gels. It is gentler, safer, and has greater compatibility with the skin when compared to other inorganic alkalizing agents (ammonia, sodium hydroxide) or other amines (diethanolamine-DEA, triethanolamine-TEA).
As it is a primary amine linked to the sugar Sorbitol (d-glucitol), Glucamine is not reactive towards N-nitrosating agents, which can nitrate secondary and tertiary amines such as TEA, and DEA, among others, leading to the formation of carcinogenic intermediates and potential irritants for older or sensitive skin. Sodium hydroxide (NaOH), an alkalizing agent often used as an alternative to TEA in gels containing ethyl alcohol, leads to the formation of a precipitate that clouds the gel. As a result of the drawbacks of normally used alkalinizers, Glucamine is a safe and effective product, which makes it possible to obtain transparent and viscous gels.
Glucamine has mild alkalizing properties and is widely used in the correction of cosmetic and pharmaceutical formulations for external use. It is used as a neutralizer in formulations such as creams, lotions, facial masks, baby care, and eye care applications.
Usually, Glucamine is used from 0.1% to 0.8% in the formulations, in amounts necessary to alkalize the acidic content. It can be added directly to the formulation in powder form, having the advantage of not altering the final viscosity, as occurs with liquid alkalizers. It is not indicated for the neutralization of alpha and beta hydroxy acids.