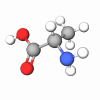
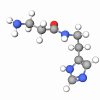
Pentapeptide-59 is a synthetic peptide derived from a part of a sequence (Arg-Arg-Arg-Phe-Val) of sea anemone (Heteractis Crispa) venom protein called APHC1. It is a perfect solution for skin sensitivity using natural mechanisms of inactivation of dermal pain receptors.
APHC1 protein might be a perfect prospect to help reduce skin sensitivity by inhibiting an overreaction of the TRPV1 pain receptor. Regardless, the complete APHC1 protein is unstable in preparation and the molecule is too big to penetrate through the skin barrier. Moreover, harvesting venom from Heteractis Crispa is not practicable for skincare products.
Therefore, a five amino acid peptide Pentapeptide-59 was designed by Mibelle Biochemistry in collaboration with the UK Venomtech Institute, who are experts in venous drug discovery. This pentapeptide repeats the amino acid sequence of the active TRPV1 receptor binding site of the sea anemone protein. Another advantage is sustainability through the synthetic production of the peptide that does not require sea anemones.
The ability of Pentapeptide-59 to inhibit TRPV1 pain receptor activation was analyzed in CHO cells that stably express TRPV1 receptors. Cells were irritated with capsaicin, which is a TRPV1 activator, either in the presence or absence of sea anemone Pentapeptide-59. Treatment with the full-length sea anemone protein APHC1 at the same molecular concentration was used as a positive control.Results showed that Pentapeptide-59 treatment inhibited TRPV1 receptor activation by 80%, whereas full-length APHC1 inhibited pain receptor activation by 25%. Therefore, the five amino acid sea anemone peptide exhibits even better TRPV1 inhibition properties than the full-length protein.