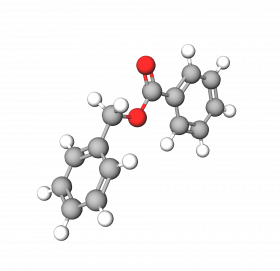
Benzyl Benzoate is an ester of benzoic acid and benzyl alcohol. It is a clear, viscous liquid at room temperature with a sweet balsamic odor. It is present in certain essential oils and fragrant substances, like jasmine, ylang-ylang, and Balsam of Peru.
Benzyl Benzoate is used in the perfume industry as an aroma fixator (to prevent the perfume scent from evaporating too quickly) and is also present in cosmetic creams. The substance is widely used in pharmaceutical products for the topical treatment of scabies in humans without any evidence of toxicity other than a very low incidence of dermatitis. Such scabicidal preparations contain levels of benzyl benzoate up to 25% w/w.
It is used as a carrier solvent for dissolving and dispersing nutrients, flavors, antioxidants, emulsifiers, and various other food ingredients and additives. In 1965, the Federal Drug Agency of the USA approved Benzyl Benzoate as suitable for foodstuffs following submissions from the Flavouring Extract Manufacturers Association. The Council of Europe set an ADI for benzyl benzoate of 5 mg kg-1 d1 in 1970.
The metabolic pathway of benzyl benzoate has been well documented: following oral administration, benzyl benzoate is rapidly hydrolyzed to benzoic acid and benzyl alcohol. Benzyl alcohol is oxidized to benzoic acid and conjugated with glycine to form hippuric acid. Hippuric acid is excreted. It should be noted that the aromatic solvent toluene is also metabolized via benzoic acid to hippuric acid. Most data on the active ingredient are from studies undertaken before international guidelines were available. They do not identify any concerns for human exposure.